- PATIENT FORMS | REQUEST A CONSULTATION | CONTACT US
- 1-844-NSPC-DOC
Symptomatic Near-Occlusion of the Carotid Artery
55 year old woman with one year of progressive hearing loss in the left ear / Acoustic Neuroma
October 27, 2021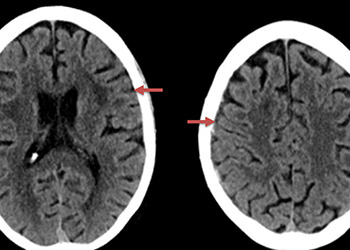
Octogenarian with mild dizziness and headache
October 27, 2021Educational Goals:
Learners will be able to recognize the symptoms that may suggest Perfusion Failure secondary to severe Carotid Artery Stenosis, and order appropriate tests to confirm the diagnosis, and appropriately refer these patients to a subspecialist for management and treatment.
A 60 y.o. woman reports awakening in the morning not feeling “normal” with gait unsteadiness, difficulty buckling a button with her left hand, and an observed left facial droop by her family. Upon further inquiry, she reported she had a similar episode of the left sided weakness approximately 3 weeks, placing her symptom onset between 9 hours and 3 weeks. Her initial NIHSS on examination was 3 with mild left facial droop with 5- power of the LUE and LLE.
On admission, her initial CT scan was negative for stroke or hemorrhage, however a CTA demonstrated severe high grade 99% stenosis of the right Carotid bulb (ICA) with markedly diminished caliber of the cervical intracranial carotid artery secondary to calcified atherosclerosis without evidence of dissection (Figure 1).
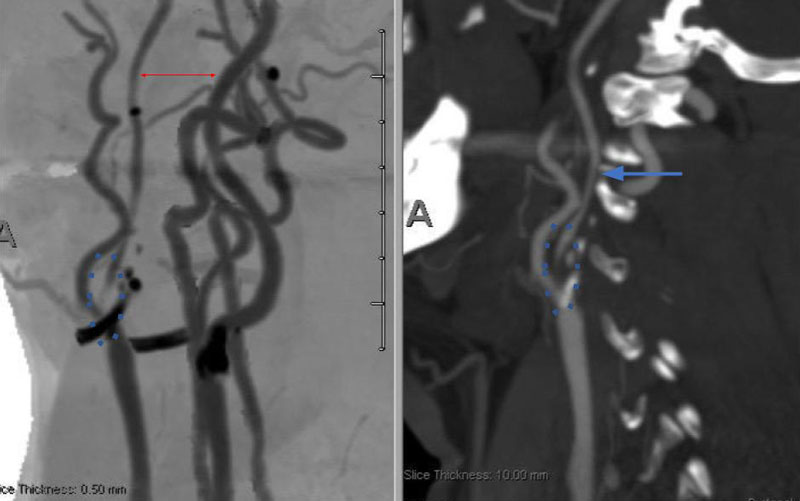
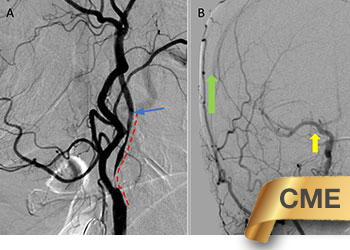
Figure 1. A and B) CTA demonstrating severe ICA 99% stenosis (dotted) with flow-reduced diminution relative to the left ICA (red arrows) and distal plaque extension to C2 level.
CT Brain Perfusion Imaging confirmed marked delay in contrast/blood Time to Peak (TMax) and Mean Transit Time (MTT) with decreased right hemispheric Cerebral Blood Flow and elevated Cerebral Blood Volume. These findings suggested that this critical right ICA stenosis resulted in significant hemodynamic perfusion failure and ischemia within the right hemispheric territories of the right MCA and ACA vascular distributions (Figure 2). No large vessel intracranial occlusion requiring emergent thrombectomy was observed, and the mild initial symptomatic presentation favored further imaging and medical optimization prior to revascularization attempts.
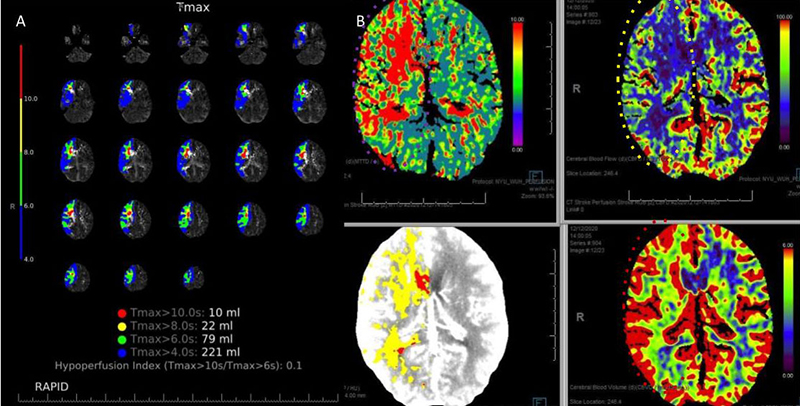
Figure 2. A) CT Brain Prefusion Tmax confirms delayed flow; B) MTT, CBF, CBV and Hybrid Threshold Parametric Maps of Right MCA/ACA revealing diminished CBF, elevated CBV, and prolonged MTT of at-risk Ischemic Tissue.
An MRI confirmed diffusion and FLAIR positive ischemic strokes within the deep periventricular white matter compatible with a “watershed” distribution of injury (Figure 3).
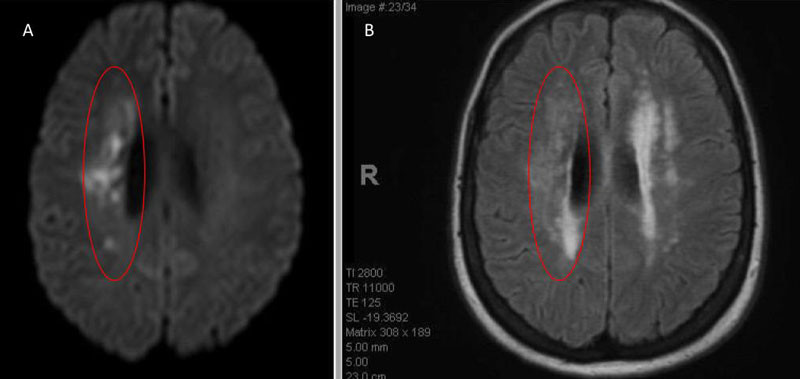
Figure 3. A) MRI Diffusion and B) FLAIR sequence confirm ischemic damage in the right periventricular deep white matter “Watershed” territories.
Catheter cerebral angiography was then performed which demonstrated the 99% stenosis with distal vascular collapse and extension of the plaque superiorly into the mid third of the cervical right ICA (Figure 4). Interrogation for collaterals from the left ICA and posterior circulations demonstrated very small and minimal contributions from the anterior communicating artery and posterior communicating artery of the Circle of Willis.
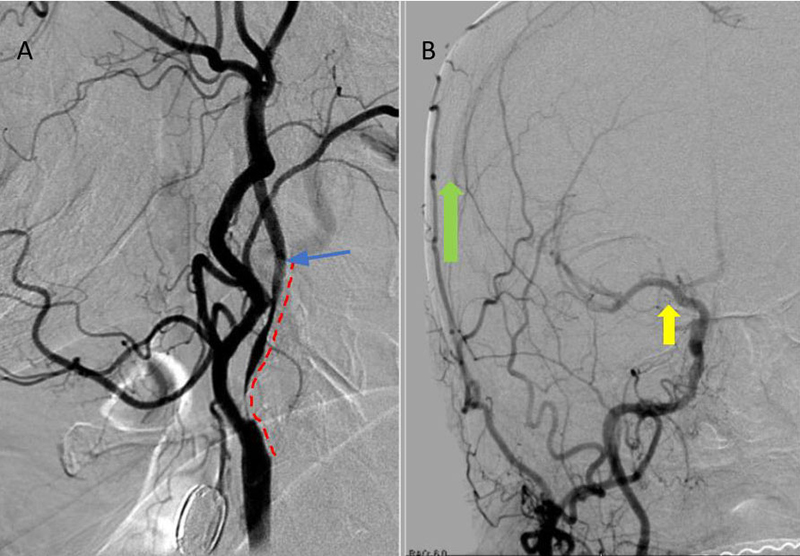
Figure 4. A) DS Angiogram demonstrates severity and extent of plaque to C2-3 level (blue). B) And marked delay of ICA perfusion (yellow) relative to ECA branches (green).
Considering extension of the plaque to the C2-3 level and the isolated circulation with perfusion failure, Carotid Endarterectomy was considered higher risk and surgically challenging. The patient was prepped with dual anti-platelet therapy and medical optimization including hydration and cardiac evaluation in preparation for Carotid Angioplasty and Stenting of her high-risk symptomatic right ICA stenosis. Contralateral stenosis of approximately 60% was also observed in the left ICA bulb.
Under Monitored Anesthesia Care, and systemic anticoagulation (ACT maintained above 250), a 6 French Guide Sheath was positioned within the distal cervical right Common Carotid Artery (CCA). Slow antegrade filling of the right internal carotid, MCA, and ACA was observed compatible with flow limitation, as well as physiologic need for this relatively isolated circulation. In addition, primary embolic protection filter placement was not considered technically feasible secondary to the severity of the “string” like stenosis. Serial primary angioplasty with 2 x 20 mm and a 2.5 x 20 mm balloon was gently performed to 10-12 ATM, creating an improved channel within the stenosis. An exchange length .014 (300 cm) “buddy” wire was then positioned across the lesion to maintain access, as an embolic protection filter was carefully navigated and deployed in the distal cervical ICA. Subsequently, a 4 x 40 mm PTA balloon was then utilized to perform angioplasty with embolic protection, followed by placement of a 40 mm stent tapering from 6 to 8 mm from distal to proximal within the right ICA and CCA. Post Stenting angiography demonstrates no significant residual stenosis with significantly enhanced right hemispheric perfusion on angiography (Figure 5).
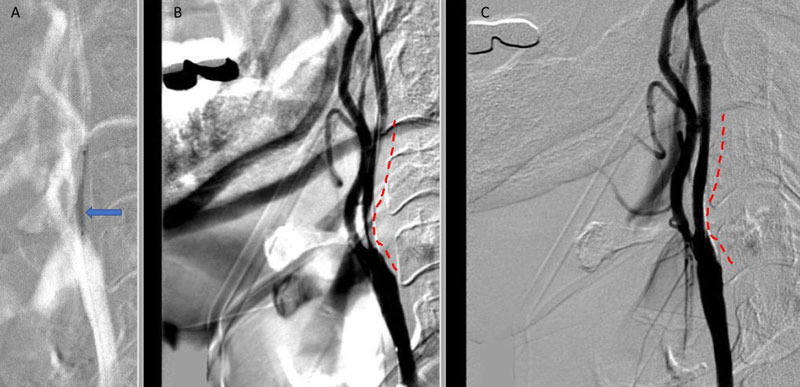
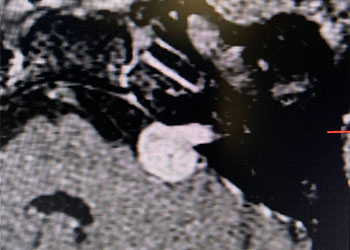
Figure 5. A) Pre-Dilatation with 2.5 x 20mm balloon (arrow) ; B) Initial PTA result with “Buddy Wire”; C) final Post PTA and Stent with no significant residual stenosis across the entire length of the atherosclerotic segment (dotted).
Post operatively, the patient did very well, subjectively feeling more alert and awake, with continued improvement in her left sided weakness and only minimal flattening of the left nasolabial fold, NIHSS of 1, on Discharge. She continues to make a complete recovery as an outpatient, having returned to all of her activities of daily living, and an mRS of 0.
Discussion:
Severe Carotid Stenosis has multiple pathophysiologic mechanisms that may resulting in mild to severe symptomatic presentations. Often, carotid-embolic events precipitate Large Vessel Occlusions of the MCA or ICA (less frequently ACA) with associated spectrum of malignant stroke syndromes based on the location, timing, comorbidities, and collateral perfusion of the patients. In our patient, the pattern of symptoms she experienced were mild and repetitive, with her deficits recovering and recurring, ultimately prompting medical attention. Her Brain Perfusion Imaging and MRI Ischemic pattern suggested a strong hemodynamic component to her ischemic injury, classically described as a “Watershed” pattern (WS). WS has often been further differentiated into Internal WS which effects the distal fields of 2 non-anastomosing arterial systems (i.e. ACA-MCA-PCA), resulting in 2 distinct WS areas: 1) in the cortical territories or Cortical Watershed (CWS); and 2) in the white matter along and above the lateral ventricle of the Centrum Semiovale – “string of pearls” termed the Internal Watershed (IWS). IWS ischemia favors a hemodynamic mechanism while purely CWS may be secondary to microembolic occlusions. A combination of IWS and CWS may indicate both hemodynamic perfusion deficits and microemboli.1 Our patient’s pattern of deep white matter involvement, angiographic anatomy, collaterals, and perfusion profile strongly favored IWS ischemia secondary to a hemodynamic perfusion failure.
Carotid Endarterectomy (CEA) has been a well-established evidence-based treatment for moderate to severe symptomatic carotid stenosis. Many trials and studies demonstrate patients with 50 to 69% stenosis, 70 to 99% stenosis, as well as patients with near-occlusions decrease the 5-year risk of any stroke or operative death compared with medical therapy.2 Certain anatomic features, such as an incomplete Circle of Willis, or an Isolated MCA (iMCA), have been reported to increase the risk of immediate neurologic events (INEs) 10-fold (24%) during cross-clamping without shunting.3 Shunting of the Carotid during Endarterectomy has been routinely and selectively used based on numerous physiologic tests and criteria, including stump pressures and TCD monitoring. Many studies have suggested that shunting may decrease the risk of INEs, however, the heterogeneity and inconsistent methodology have limited broadly generalizable conclusions.4 Our patient, in addition to high risk physiologic pattern (iMCA), had extension of her atherosclerotic plaque distally into the high-cervical C2 region, which presents a technical surgical challenge for distal control of the vessel during surgery requiring more extensive dissection and in some situations, mandibular subluxation5,6. After extensive consideration of her presentation and risk factors, we strongly favored an endovascular approach.
Carotid Angioplasty and Stenting has become a widely accepted alternative to CEA, with many trials demonstrating risks of peri-operative stroke, comparable to CEA in “Standard” Risk patients and perhaps favored in high-risk presentations, well below the recommended 6% (symptomatic) and 3%(asymptomatic) thresholds for risk-reduction benefits compared with medical therapy for moderate to severe Carotid Artery Stenosis.7,8 As discussed, several high-risk variables in our patient favored an endovascular approach to mitigate peri-operative risks of ischemia and more extensive dissection. Symptomatic ICA Near-Occlusions also represent a technical challenge for endovascular approaches, however, have been shown to have comparable rates of peri-operative complications, restenosis, and vessel maturation to CEA in the available published experiences9. Our technique of gentle pre-dilatation with a submaximal smaller diameter balloon (2.0-2.5 mm), followed by an embolic protection filter, therapeutic angioplasty, and stent placement has been employed successfully with low-rates of immediate adverse events and long-term durability.
In conclusion, Carotid Revascularization for Symptomatic Carotid Disease is a well-established efficacious treatment to decrease the patient’s risk of future neurologic events, morbidity, and mortality. The choice of revascularization techniques has evolved over the last decade and affords a multi-disciplinary experienced team to choose the best strategy for each individual patient’s presentation, anatomic and physiologic features, and risk factors.
References:
- Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 2005;36(3):567-577. doi:10.1161/01.STR.0000155727.82242.e1
- Rerkasem A, Orrapin S, Howard DP, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2020;9:CD001081. Published 2020 Sep 12. doi:10.1002/14651858.CD001081.pub4
- Banga PV, Varga A, Csobay-Novák C, et al. Incomplete circle of Willis is associated with a higher incidence of neurologic events during carotid eversion endarterectomy without shunting. J Vasc Surg. 2018;68(6):1764-1771. doi:10.1016/j.jvs.2018.03.429
- Chongruksut W, Vaniyapong T, Rerkasem K. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev. 2014;2014(6):CD000190. Published 2014 Jun 23. doi:10.1002/14651858.CD000190.pub3
- Kondo T, Ota N, Göhre F, et al. High Cervical Carotid Endarterectomy-Outcome Analysis. World Neurosurg. 2020;136:e108-e118. doi:10.1016/j.wneu.2019.12.002
- Frim DM, Padwa B, Buckley D, Crowell RM, Ogilvy CS. Mandibular subluxation as an adjunct to exposure of the distal internal carotid artery in endarterectomy surgery. Technical note. J Neurosurg. 1995;83(5):926-928. doi:10.3171/jns.1995.83.5.0926
- Müller MD, Lyrer P, Brown MM, Bonati LH. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst Rev. 2020;2(2):CD000515. Published 2020 Feb 25. doi:10.1002/14651858.CD000515.pub5
- Cole TS, Mezher AW, Catapano JS, et al. Nationwide Trends in Carotid Endarterectomy and Carotid Artery Stenting in the Post-CREST Era. Stroke. 2020;51(2):579-587. doi:10.1161/STROKEAHA.119.027388
- Kim J, Male S, Damania D, Jahromi BS, Tummala RP. Comparison of Carotid Endarterectomy and Stenting for Symptomatic Internal Carotid Artery Near-Occlusion. AJNR Am J Neuroradiol. 2019;40(7):1207-1212. doi:10.3174/ajnr.A6085
Disclosures:
The planners and faculty participants do not have any financial arrangements or affiliations with any commercial entities whose products, research or services may be discussed in these materials.
CME Accreditation:
This activity has been planned and implemented in accordance with the accreditation requirements and Policies of the Medical Society of the State of New York (MSSNY) through the joint providership of the Academy of Medicine of Queens County and NSPC Brain and Spine Surgery. The Academy of Medicine of Queens County is accredited by The Medical Society of the State of New York (MSSNY) to provide continuing medical education for physicians. The Academy of Medicine of Queens County designates this Enduring Materials for a maximum of 1 AMA PRA Category 1 Credits™ as specified in this activity. Physicians should claim only the credit commensurate with the extent of their participation in the activity.